In Wednesday’s newsletter I invited readers to email me their questions regarding the COVID-19 vaccines. As people are weighing the decision to be vaccinated when it’s their turn, I have found that a lot of the hesitancy stems from not having a place to ask questions and get answers. So today’s newsletter will be devoted mostly to answering some of the more common questions I’ve received since Wednesday. I likely won’t be able to answer all of them today, but will likely make next Friday’s newsletter a Vaccine Q&A edition also. Toward the end of today’s newsletter there will be a very brief highlights section for the latest Georgia numbers.
General comments and expectations
At the outset, I should remind all of my readers that I am not a medical doctor and my words today should not be considered medical advice. In addition, each person’s medical history is different and unique to them. Your medical decisions are meant to be a team effort between you and your physician with your consent. So if you have specific medical questions about your unique situation, then I recommend asking those questions to the person who knows your medical history. My comments here are intended for a broader audience. They are an effort to “translate” the science and the medical knowledge into a format that is easily understandable for members of the general public. This is sort of a role that I have filled for friends and family for years. For whatever reason, they don’t think they’re getting satisfying answers from their healthcare team. Sometimes that’s a perceived lack of time, patience or interest from their provider (sometimes justified, sometimes not). Sometimes it’s providers putting the decision back in their hands and they need to sound out the pros and cons. Sometimes it’s fear of looking stupid and instead asking me the questions they’re afraid to ask their own doctor - because we have some sort of a bond (friendship, family, etc) and they know I’m not going to laugh at them or judge them. So as I thought about writing today’s newsletter this morning, this history is what I hope to continue here today. The message below from one of my readers really made me think hard on my goal of being sensitive to public need and seeing the gaps between how scientists talk to each other versus how we talk to the public.
“But in searching for expert opinion one receives only a pat on the head and assurance that everything will be all right…The over-riding impression is that politicians and media are driving public towards taking the shots without solid information about the risk involved.”
I, too, have experienced the “pat on the head” and had my own questions ignored in the past. I know what you’re talking about. Where the data may be obvious to us scientists, it’s not necessarily to the public. And authority is meaningless if the relationship and trust aren’t built first. I think it’s a good thing that you’re asking questions and wanting data. I want you to be and feel heard. I want to find the data that pertain to your questions. I want you to make data-driven decisions in all areas of your life.
Be careful to make sure you’re using trusted resources to make decisions. Remember when we used to hear that you can’t believe everything you read? Well that extends to the internet as well. People can put whatever they want on the internet with little fact-checking or verification. Be wary of things you read on social media or see on YouTube for this reason. Also be wary of anecdotal evidence - we tend to focus most on the stories of a few, rather than the entire body of evidence. On that same token, I want you to look at *my comments* through this same skeptical lens. You and I may have a history together - perhaps you know me in real life or you’ve been reading my newsletters since the beginning. I still want you to always make sure that the data make sense to you, that you know how to read them, and that you can form your own conclusions. My most trusted resources public health data themselves are the Department of Public Health (Georgia), the CDC, FDA, World Health Organization and the US Department of Health and Human Services. I also spend a lot of time in the science and medical literature - peer-reviewed articles and studies. For COVID-19, my favorite journals have been the New England Journal of Medicine, the Journal of the American Medical Association, and the Morbidity and Mortality Weekly Reports (put out by CDC). A lot of government agencies work to make those peer-reviewed studies more relatable for non-scientists. They include the Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), the National Institutes of Health (NIH), the National Science Foundation (NSF) and others. Sometimes, the science and medical journals will do this work too with helpful commentaries and graphics. In this pandemic, however, we’ve seen non-government agencies and individuals step into the role of science communication when government-driven science communication was lacking. Just be careful to make sure the person doing the talking is qualified to be speaking on that subject (i.e. they have a background in public health or infectious disease) and pay attention to what the majority of scientists you’re hearing from are saying. If scientists are all skeptics and using the scientific method in our analyses, then hearing the same thing independently corroborated by others is a GOOD sign that you’re getting good science communication. Scientists typically aren’t afraid to call each other out if we see a logic flaw - it’s how peer-review works.
People understandably have a lot of fear and anxiety about a variety of things since the pandemic began. Living through history is chaotic and stressful and there are a lot of things we don’t fully understand - about the virus, the timeline of getting back to “normal,” etc. What tends to happen is that the things we don’t know or don’t understand give us the most anxiety. The vaccines are just the latest trigger for anxiety for many people. There are some things we don’t yet know, just because the pandemic and the vaccines are relatively new still. However, my hope is to assuage the concerns for which we do have data and do have reassurance. I’m not here to pressure you to get the vaccine but to make sure you have the best data and science that we have right now to make the most informed decision and feel confident in doing so - either way. My goal is to empower you to make data-driven decisions, as I have since the beginning of the pandemic.
Was the process of development rushed?
I’ve previously written on this topic here. The short answer is that the vaccines went through the same safety and efficacy checks that would happen during non-emergency FDA approval. Whereas traditionally there are stops where data are submitted to FDA at interim steps and approval is then granted for progression to further trials, the process is streamlined in this emergency situation. They submit data to FDA on a regular basis but the clinical trials are staggered and more efficient. In addition, because it is an emergency, the vaccine manufacturers applied for emergency use authorization (EUA) prior to full FDA approval. This is due to the emergency nature of our situation - lots of people are dying and we have at least two vaccines so far that prevent death. Data will continue to be collected on clinical trial participants to gauge how long immunity lasts, how well the vaccines interrupt transmission, and continued safety data in all demographic groups for about two years prior to FDA approval. The emergency timeline of EUA is the fastest that we can still carefully study a potential vaccine or drug to ensure its safety and efficacy.
How were the phases of eligibility determined?
The phases of eligibility were determined by the Advisory Committee on Immunization Practices, a group of 15 medical and public health researchers from universities across the country and includes one consumer advocate. No one from the pharmaceutical industry can serve as a voting member. They hear presentations from people at the CDC and consider various pieces of evidence and data before recommending and setting the dosing schedules for vaccines for children and adults, most recently including the COVID-19 vaccines. They weigh the risks and benefits of administering a vaccine versus the disease itself. They also look at efficacy data to see how close the intervals should be between doses to see what will work best for preventing disease. They weigh when a person should be vaccinated - under what conditions, with or without underlying conditions. In addition, what is the ideal range of time to immunize someone against a threat considering demographic data? For example, let’s administer the shingles vaccine before immune system decline begins at age 65 (when virus reactivation is more likely to occur). Let’s vaccinate children for HPV *before* they become sexually active. Let’s vaccinate incoming college students for meningitis *before* they live in the dorms.
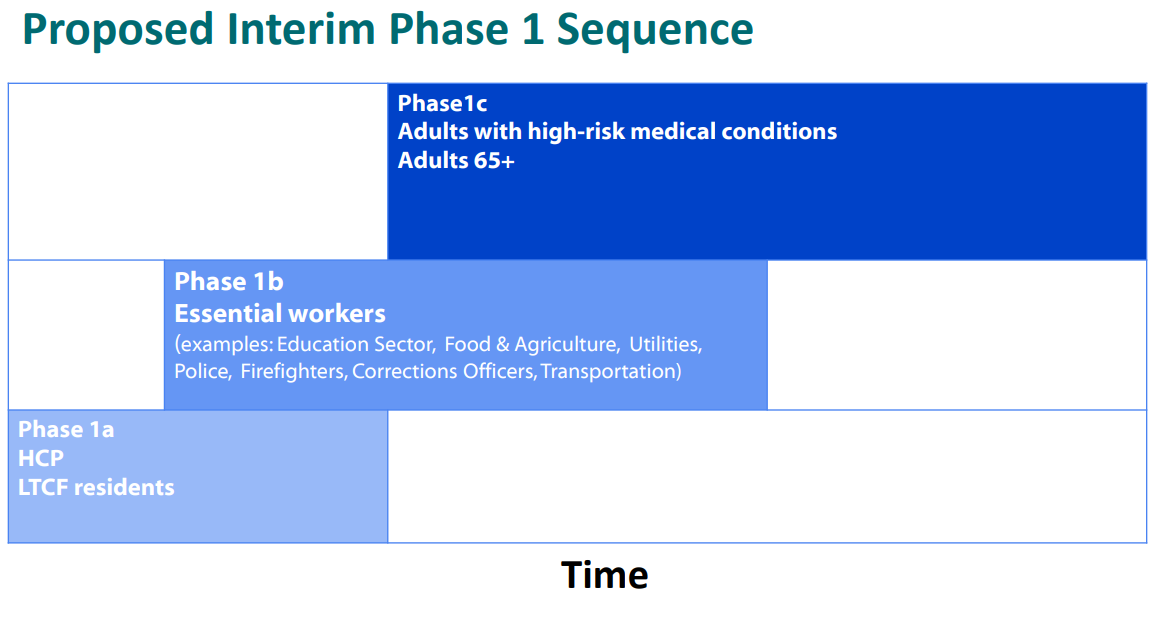
They discussed the phased allocation of COVID-19 vaccines in two meetings - on November 23rd and December 1st. Knowing there would be a lot more people who would want the vaccine than there would be doses, they sought to achieve a balance between science, implementation and logistics, and ethics. Another way to phrase this could be who is being most harmed by the pandemic, who can we help the most, and how do we preserve our ability to care for the sick? Given that deaths are occurring most among people >75 years of age and especially those who are admitted to the hospital from a long-term care facility (LTCF), they decided these people should be in Phase 1a. Because healthcare workers are vital to maintaining our ability to care for the sick and because they have an enormous risk of exposure, they were also included in Phase 1a. Phase 1a is aimed at preserving life and hospital capacity.
A second question I’ve received is how many people are in the eligibility categories, sort of as a way to gauge where we are in the process. So we’ll consider that as we discuss the other categories too. Here’s one of the ACIP slides from the 23Nov2020 meeting that estimates those populations on a national level. The initial recommendation to vaccinate healthcare workers and LTCF residents and staff in Phase 1a meant vaccinating ~24 million people, 21 million of whom where healthcare workers, 3 million of whom were seniors living in congregate settings such as a nursing home.
They went on to propose that Phase 1b focus on essential workers, including educators, first responders, food and agriculture, transportation and utilities staff. This phase is aimed at protecting people with the greatest risk of exposure who keep our society running. What good is it to survive COVID-19 but starve or freeze to death? People can’t work (or work well) when their kids aren’t in school. The estimated size of this population in the US is 87 million people.
Okay, by phase 1c, we’ve addressed how we survive the most brutal aspects of the pandemic (deaths and overwhelming hospitals) and keeping society’s basic needs met. In Phase 1c, the priority becomes protecting people with high risk medical conditions and those 65+ who do not live in congregate settings were recommended to receive the vaccines. The estimated size of these populations in the US are 100 million and 50 million people, respectively. These people are at a high risk for more severe outcomes of the disease, but may have greater capacity to shelter away from it than healthcare workers, LTCF residents and essential workers.
States have chosen to follow, modify or not follow the ACIP recommendations. Georgia is among the states that have chosen to modify the recommendations, moving people from the 65+ who do not live in congregate care to phase 1b with first responders and then moved other essential workers (such as educators, food and agriculture, utilities and transportation) to phase 1c along with adults with high-risk medical conditions.
At present, the US has administered vaccine doses to at least 35.8 million people. If the ACIP recommendations were being followed nationwide, that would be enough to complete phase 1a and enough to vaccinate 11.8 million (13.6%) of phase 1b. However, with the eligibility criteria varying so widely across states, it’s kind of hard to know where we stand. States also have their own gating criteria for when to progress to the next phase. You can read Georgia’s phased allocation plan here. I think it would help a lot for state residents to see a thermometer or fuel gauge inspired graphic that shows us where we are in the process. I think many of us understand that we might have to wait our turn. The hardest part of all of this is not knowing where your place is in line.
There are a lot of hurt feelings with these phase recommendations and the ways that states have chosen to modify them. It sounds as though vaccine supply is going to improve dramatically in the coming months. Hopefully that will mean that we will progress through these phases more quickly than we are now.
How do the vaccines work?
I’ve written on this topic previously, so I’ll refer you to some links below.
How the immune system works (a simplified crash course)
A crash course on cell and molecular biology and how the RNA vaccines work
Efficacy data for the Pfizer and Moderna vaccines
The DNA vaccines from Johnson and Johnson and AstraZeneca, also a discussion of how we anticipate the vaccines will do against the variants and some information on vaccine trials taking place for children.
Comparing the efficacy of the RNA vaccines versus the Johnson and Johnson vaccine
Future topics
As you can imagine, I’ve received A LOT of questions. Today I focused on the easiest ones to answer and setting expectations. I’ve organized the questions into topic areas that I will address in upcoming newsletters:
Our path back to “normal.”
Vaccine logistics including why two doses are required and what to expect in terms of reactogenicity (expected, manageable side effects).
How long does immunity last?
Long term effects of the vaccines
Adverse reactions
Special populations and their risks and benefits of vaccination: autoimmune disorders, people with a history of allergies, pregnant/lactating women, and those who are trying to conceive
The list above does not indicate any rank of importance or the order in which I will answer them. I just want to let you know what’s coming in case you have a similar question. If you have a question that doesn’t align with the topics listed above, please email me.
Please be patient if I didn’t get to your question today. For some of these, we don’t have a lot of data yet and I have to search for them to give you the best that current science has to offer. And I’m also limited for time and space in these newsletters. In the meantime, for what it’s worth I will tell you that I plan to take whichever vaccine is made available to me first as soon as I am eligible. That is my level of trust in the vaccines and my level of seriousness when it comes to the virus. It’s okay if that’s not your level of trust or seriousness at this point. It’s okay to ask questions and want answers. I will get to them as soon as possible. But perhaps my personal view can provide some reassurance in the meantime - I would not suggest you do something that I would not do for myself or my children.
I appreciate your trust in me to answer your important questions. Thank you for your patience!
Georgia highlights
Testing: 27,070 new PCR test results today, 8.8% of which were positive. Antigen testing identified 37% of today’s newly reported cases. We are likely going to see a big drop in weekly test output this week when I do the analysis on Sunday.
Cases: net increase of 3900 newly reported cases (2456 by PCR, 1444 by antigen test). The state’s 7-day case rate per 100,000 is now 5% below the summer peak, but 219% above the pre-winter surge baseline.
Hospitalizations: there were 258 new COVID-19 admissions to the hospital and 24 new admissions to the ICU. According to the HHS, there are 3564 patients currently hospitalized for COVID-19 including 62 children. All ICU beds in hospital region A are full, for the second day in a row.
Deaths: We unfortunately set a new record today for newly reported confirmed COVID-19 deaths, at a net increase of 184 compared to yesterday, plus 11 probable deaths.
References
https://healthdata.gov/dataset/covid-19-reported-patient-impact-and-hospital-capacity-state
https://dph.georgia.gov/covid-19-daily-status-report
https://covid-gagio.hub.arcgis.com/
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/COVID-02-Dooling.pdf
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-11/COVID-04-Dooling.pdf
https://www.cdc.gov/vaccines/acip/members/index.html
https://covid.cdc.gov/covid-data-tracker/#vaccinations
https://dph.georgia.gov/document/document/georgia-covid-19-vaccine-plan/download
https://www.cbsnews.com/video/biden-administration-secures-200-million-additional-vaccine-doses/
https://amberschmidtkephd.substack.com/p/the-daily-digest-11dec2020
https://amberschmidtkephd.substack.com/p/the-daily-digest-18dec2020
https://amberschmidtkephd.substack.com/p/the-daily-digest-16dec2020
https://amberschmidtkephd.substack.com/p/the-daily-digest-29jan2021
https://amberschmidtkephd.substack.com/p/the-daily-digest-03feb2021
Georgia COVID-19 Updates is a free newsletter that depends on reader support. If you wish to subscribe please click the link below. There are free and paid options available.
My Ph.D. is in Medical Microbiology and Immunology. I've worked at places like Creighton University, the Centers for Disease Control & Prevention and Mercer University School of Medicine. All thoughts are my professional opinion and should not be considered medical advice.