The Daily Digest, 04Dec2020
Georgia COVID-19 Updates
As I mentioned in the last newsletter, we’re going to discuss some of the intricacies of the coronavirus vaccines over the next few weeks. The information can be overwhelming, so I want to lay things out in pieces. Last time, I talked about the Advisory Committee on Immunization Practices, a group of 15 voting members (one from CDC, the rest from academic institutions across America, including one consumer representative) who go over safety data and set the dosing schedules and vaccine policy for the United States. This committee sets the vaccine schedule for pediatrics as well as adult immunizations. Today we’re going to talk about how vaccines and other therapeutics are evaluated and how they progress through the FDA approval process. In other words, it’s the work that had to be done before applying for Emergency Use Authorization by the FDA and before ACIP sets the dosing schedule and policy. As a follow up, the New York Times published this possible timeline of when different populations might be eligible for vaccination over the next year. This second interactive tool from NYT estimates where you fall in the line of people waiting for a vaccine based on the county where you live, the type of job you have, your age and if you have any of the medical conditions that make one more vulnerable to severe cases of COVID-19.
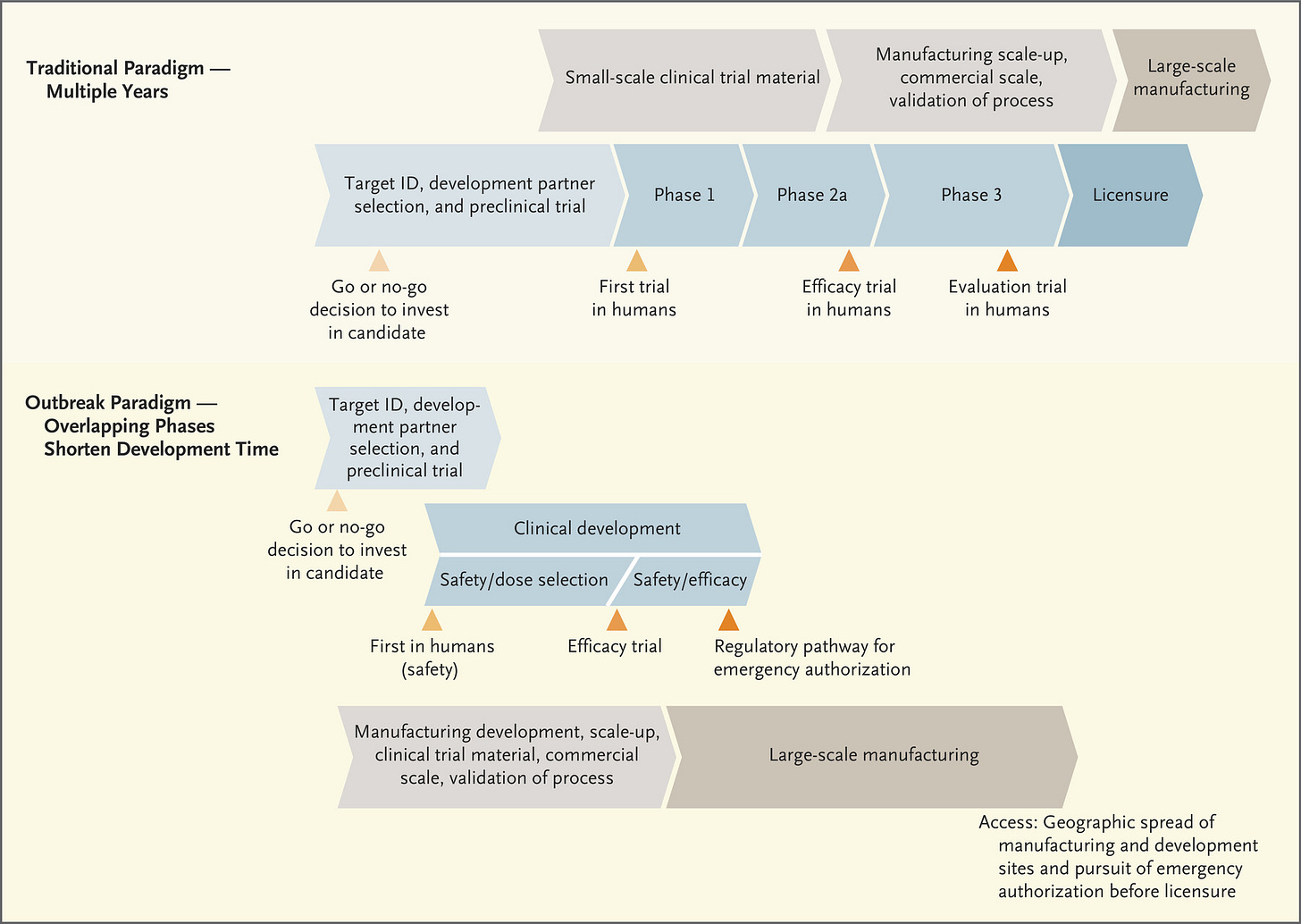
The figure below lays out how the FDA approval process typically works, when we’re not experiencing an emergency. A lot of it begins with basic science in research laboratories. “Basic” doesn’t mean simple or even cheap - it studies how living things work at their most fundamental levels - the interaction of cells, proteins, nucleic acids, and other biomolecules. The research they’re doing often doesn’t have an immediate clinical impact on human health. For example, I used to teach about researchers in Antarctica who were studying the composition of cell membranes (the cell borders) in fish that live in very cold temperatures. What does a cold water fish’s membrane composition have to do with human health? Well, it turns out that the way those cell membranes keep from freezing and locking into place (disrupting cross-membrane transport of nutrients, etc) is by inserting more cholesterol (and related molecules) into their membrane. To do this, they deliver cholesterol to the cells via the bloodstream. How do these fish maintain cardiovascular health when we know that high cholesterol in the bloodstream is detrimental to cardiovascular health in humans? What mitigation strategies do they use and can we use something similar in a therapeutic for humans? So, you see, the basic science is very specific. But it is also iterative and collaborative. As more information is learned about the intersection of molecular biology, chemistry and physiology in living things, the information builds on itself and we connect the dots and solve problems. But this process can take years, which is why it is so critically important to fund basic science investigations robustly and over the long term. All of this effort feeds into the early stages of the timeline shown below.
One big thing I want you to take away from the diagram above is the TIME that it takes to go through this process, and it assumes that everything goes well as the process goes on. The process slows or stops if problems arise. Also pay attention to the fact that safety is a feature of all three phases of the clinical trials, gradually increasing the population size of people or animals in the studies to verify safety. Phase I focuses exclusively on safety - can we administer the vaccine/therapeutic and see minimal (if any) side effects? Phase II continues to look at safety, with a larger population, and now checks for immunogenicity or the ability to generate an immune response. Do the trial participants make antibodies in response to the vaccine? If they see that it does, in fact, result in antibody production and is still safe, then they progress to Phase III. In Phase III, the population size increases further. But now that we know that people who receive the vaccine produce antibodies, it’s time to test efficacy - does the vaccine, and the antibodies test subjects are producing, actually protect people from being infected with COVID-19? Along the way, data are being submitted to the FDA as each phase is being conducted. In the next diagram, the traditional paradigm section is more or less the same as what you see in the figure above, but it adds the manufacturing process in gray/tan. You’ll notice that large scale manufacturing begins only after the FDA licenses the vaccine or therapeutic.
But these aren’t ordinary times. We can’t remain in this societal state of suspended animation for 10+ years waiting for the COVID-19 vaccine. So the process is streamlined during public health emergencies. It’s important to note that corners are not being cut by expediting this process - the safety and efficacy checks are still happening and still with large population samples. But it’s less iterative - you can move to the clinical development in humans, for example, as you’re continuing to gather data during pre-clinical trials (i.e. in laboratory animals). The other thing that really distinguishes this timeline from the traditional timeline is that the manufacturing process begins right away, this is “Operation Warp Speed” as the US government has named it. This means pretty big investment in a number of vaccines as they’re being developed, even knowing that some of them won’t ultimately work or meet FDA approval. But the idea is that the investment is worth it to get working/approved vaccines mass produced for the public so they can be administered as soon as we have approval.
Another arm of “Operation Warp Speed” is not just the manufacturing but the distribution of those vaccines as they come off the line. But the third thing we need is to educate the public and get public buy-in to taking the vaccine, to the tune of >70% of the population in order to achieve herd immunity. That requires building public trust in the process of vaccine development, and that is what I’m trying to accomplish by teaching you how vaccines work and how they’re tested. People fear what they don’t understand. If we can understand better, then the anxiety might diminish, replaced instead with confidence.
That’s it for today on vaccines. Next time, we’ll dig a little deeper into what antibodies are and how our immune systems respond to vaccines, including why all three of the COVID-19 vaccines applying for EUA from the FDA require two doses.
Testing
Now that the month of November is over, we can revisit how well the state is doing at testing its population and how test rate and percent positive vary depending on county type. For these analyses, they are based only on PCR testing because those are the only data that the Georgia Department of Public Health has provided. First, let’s begin with a look at how percent positive rate has changed over time. For each of these months, the data used are based on date of test report. The black line in the graph below shows the statewide average. You can check to see how I classify your county by referring to the table in this post. The goal is to be at or below 5%.
The state’s percent positive rate rose in the month of November, from 6 to 8% (or an increase of 33%). The best performing county type is that of Atlanta (counties of Fulton and DeKalb). Atlanta suburbs have performed more or less along with the statewide average since July. But nonrural and rural counties have had higher positive rates. As we see the percent positive rate increase, that signals that we are likely missing cases that contribute to ongoing disease transmission. That also means that the case rate for counties with higher percent positive rates are likely an underestimate of the situation.
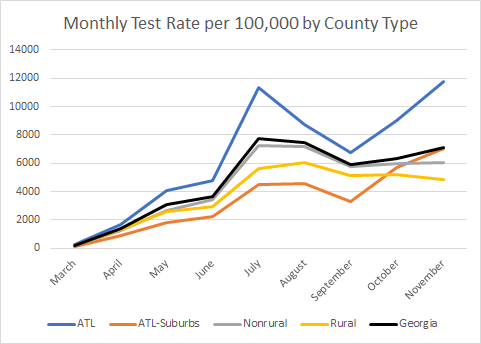
So if we are worried that we might be under-counting cases, then it makes sense to look at how well each county type is doing at testing its population (see the graph below). The black line in the graph below reflects the statewide average test rate.
It’s perhaps not surprising that the Atlanta counties, with their low percent positive rate, are accomplishing this with a really high test rate. In fact, their test rate is 66.5% higher than the state average. Nonrural counties test at approximately the same level as the statewide average but their test rate has remained flat over the past two months. And Atlanta suburb counties, that have historically underperformed relative to the state average, have increased their test rate dramatically (+115%) since September. Rural counties have consistently tested their population at a rate that is below the state’s average since the beginning of the pandemic and the gap is widening. Whether this is due to lack of awareness or availability or due to attitudes toward the seriousness of the pandemic is unclear.
Remember how I said that it’s important to test widely enough that we’re not missing cases? It’s therefore useful to compare test rates with case rates. The greater the difference between test rate and case rate, the better. For the month of November, you can see how these rates compared for each county type.
Because of the scale of the y-axis, it’s hard to see more subtle differences in the case rate for each county type. So I’ve provided the table of data below the bars. The case rate is lowest in Atlanta counties and the test rate is highest. So their delta (or difference) between the two metrics is the largest. Atlanta suburbs are very comparable to the state average. But the narrowest delta between test rate and case rate is for rural counties. As a reminder, we do not get to see the impact that antigen tests are making on test rate. It’s entirely possible that rural counties are doing much better if antigen tests are included.
In today’s update from the Georgia Department of Public Health, there were 38,355 newly reported PCR test results (a medium-high day for us). Of those, 12.2% were positive and that is part of a trend over the past week or so. The state does not provide data to the public regarding the number of antigen tests performed nor the percent that are positive.
Cases
Using both PCR and antigen test case data, our 7-day case rate per 100,000 (252) has now exceeded where we were at the height of the summer surge (214 cases per 100,000). In other words, our 7-day case rate is now 18% higher than what we witnessed at our worst point in the summer. Today there was a net increase of 4947 PCR cases and 1429 antigen cases compared to yesterday’s totals, for a combined total of 6376. This is the highest single day increase we’ve seen, breaking the record set on 21Nov (6209 combined total). You can see how case rates are moving over time for each county type in the graph below. Ignore the big spike in early October - that’s when Georgia DPH let us know about 26,000 antigen cases all at once.
Here’s an updated look at the Harvard Global Health Institute-inspired map adjusted to include Georgia’s antigen cases. A reminder, the HGHI uses whatever Georgia reports to USAFacts.org and Georgia chooses to only report PCR cases. Click on the map below to see a live version where you can click or hover over your county of interest.
This week’s school aged surveillance data contains data up to 02Dec, so the most recent week’s worth of data in the report includes 4 days’ worth of holiday and weekend effect. So despite the fact that some of the graphs currently show decreases, I would not anticipate those trends are real, but an artifact of laboratory closures. The graph below shows the case rate (all tests, PCR + antigen) per 100,000 for different age groups.
The trend is showing increases for all age groups (again, I don’t put much faith in the most recent week’s data). The other thing to notice is that the case rate is higher (adjusted for population) among 18-22 year olds compared to any other age group, but older adults are not far behind.
Hospitalizations
Yesterday, I posted some “breaking news” sort of updates that I felt needed to be communicated prior to today’s newsletter so people could react and plan. Click on the link below to see the entire thread. I do not think you have to have a Twitter account to see it. Keep in mind that the concerning trends we are seeing now do not even reflect the consequences of Thanksgiving gatherings yet. There are worse conditions yet to come.


And I am not the only one sounding the alarm or signaling the significance of what is happening and what is expected to transpire in the coming weeks. Yesterday, the state’s Insurance and Safety Fire Commissioner, John F. King, issued a directive for insurance companies to expedite hospital discharge authorizations to nursing homes or LTCFs (lower levels of care than a hospital) to free up space in hospitals. A similar directive was issued at the start of the Georgia pandemic in March.
For those who keep asking how ICU bed use compares to usage rates a year ago: when the beds are full and it is your loved one who is not receiving optimal care due to limited staffing and resources, I’m not sure that it will matter what ICU usage rates looked like last December. The problem is lack of availability NOW. The usage rates we are seeing now are higher than what we have seen during “quiet” periods of this pandemic when we weren’t experiencing a surge.
Today Georgia recorded a net increase of 212 new hospital admissions and 43 admissions to the ICU. Whereas past weeks have mostly seen daily numbers in the 100s Monday - Friday, this week every day so far has been in the 200s. There are 2366 patients currently hospitalized for COVID-19, an increase of 66 compared to yesterday’s total. 85% of the state’s ICU beds are in use with >90% use in hospital regions C (91%), E (99%), H (91%), L (97%) and N (97%). Adult ventilator use is trending up slightly with 9 days at 31% that are driving the 7-day average up.
Another important figure from the School Aged Surveillance Data Report is the one below showing how ER visits for COVID-19 disease are trending for pediatric and college aged patients.
Note that ER visits are increasing for every age group, with tight agreement among those 11-22.
Deaths
There was a net increase of 43 newly reported confirmed deaths today, a medium-low day for Georgia. The updated total is 8922. We will likely cross 9000 by the early part of next week if trends continue as they are. Nonrural counties led with 15 deaths and rural counties were close behind with 14, not that this is a competition that anyone should want to win.
In closing
The scary things we see in the hospital data today don’t include the anticipated surge that’s expected as a consequence of Thanksgiving gatherings. Next week will most likely be worse as well as the weeks to follow. As much as we’d all prefer that people would just do the right thing when we ask them to, it’s clear that the strategy and messaging isn’t working. We are living through the pandemic version of The Hunger Games right now. People do not care that their actions might hurt others. In fact, some seem to delight in it. We need government to step in, just as they do when the actions of individuals hurt others (i.e. DUI laws, no smoking inside of restaurants or public buildings, etc). To be abundantly clear, I’m not asking for intervention that lasts forever, but we have to do something for the short term. If we push our healthcare infrastructure to the point of breaking, we will see a lot of people die who could have been saved under normal circumstances - for all sorts of conditions, not just COVID-19. When the beds are full and the staff is short, well…may the odds be ever in your favor.
Please make good choices.
References
Traditional vaccine development timeline: https://www.nejm.org/doi/full/10.1056/NEJMe2025111
Developing vaccines at pandemic speed: https://www.nejm.org/doi/full/10.1056/NEJMp2005630
Who and what is the Advisory Committee on Immunization Practices: https://www.cdc.gov/vaccines/acip/members/index.html
NYT estimate timeline of vaccine eligibility: https://www.nytimes.com/2020/12/02/briefing/pfizer-vaccine-elliot-page-trump-children.html
NYT interactive tool to find your place in line for a vaccine: https://www.nytimes.com/interactive/2020/12/03/opinion/covid-19-vaccine-timeline.html
Georgia Office of Insurance and Safety Fire Commission directives archive: https://oci.georgia.gov/press-releases/directives
School Aged Surveillance Data report: https://epidemiologyschoolreport.s3.amazonaws.com/SchoolAgeSurveillance1123.html
Georgia Daily Status Report: https://dph.georgia.gov/covid-19-daily-status-report
Georgia Geospatial Information Office Data Hub: https://covid-gagio.hub.arcgis.com/
Georgia COVID-19 Updates is a free newsletter that depends on reader support. If you wish to subscribe please click the link below. There are free and paid options available.
My Ph.D. is in Medical Microbiology and Immunology. I've worked at places like Creighton University, the Centers for Disease Control & Prevention and Mercer University School of Medicine. All thoughts are my professional opinion and should not be considered medical advice.