Johnson and Johnson vaccine
As discussed on Wednesday, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) is meeting today to discuss the clinical trial data for the Johnson and Johnson vaccine (AKA Janssen) and recommend it for Emergency Use Authorization. I wanted to take some time today to unpack some of the key data that are in the briefing document. You can review similar documents for Pfizer and Moderna’s vaccines. Each of these briefing documents are the FDA’s review of the clinical trial data that were submitted by the manufacturers with special focus on both efficacy (how well the vaccines work) and safety. We also get to see the demographic data for who was in the clinical trial, including age, sex, race and ethnicity, and underlying conditions.
I’d like to first begin by showing you the incidence curves for the different manufacturers. The x-axis shows time since the first dose and the y-axis shows cumulative incidence so new infections of COVID-19 for the trial participants. The length of the study in these graphs is similar. The color coding isn’t consistent here, but the line that goes from lower left to upper right in all the graphs is the placebo group. This means they did not receive the vaccine, but something harmless like saltwater solution. The blue line (Janssen or Pfizer) or red line (Moderna) shows the vaccine group. The graphs on the right for Moderna and Pfizer were looking at incidence of infection in general (wide spectrum of severity). In contrast, the graph for Janssen (left) is looking at incidence of infection that was moderate to severe/critical. So it isn’t a straight apples to apples comparison.
What we see here is that the Moderna and Pfizer vaccine recipient lines diverge from the placebo group starting about 10 days after the first dose and then they stay flat for the duration of the study. For the Janssen curve, the vaccine and placebo groups diverge around day 14 and while the difference widens over time, it’s a narrower difference than we see for the other vaccines. Part of this may be explained by the fact that a booster dose is given in the Moderna and Pfizer trials, keeping incidence (this word means new disease) low.
It’s important to note that none of these are challenge trials where we inject people with either vaccine or placebo and then deliberately expose them to COVID-19 to see who gets sick and who doesn’t. We can do those kinds of studies in laboratory animals but in humans that is fraught with ethical conflict in a situation such as this where the disease has the mortality rate that it does. Instead, in these trials people were vaccinated (or not) and then lived their lives as the rest of us have in the pandemic. Some might be taking more risk than others. But since nobody knew whether they received vaccine or placebo, we can assume that variations in risk tolerance were equally represented in both groups. The vaccine manufacturers also weren’t doing active surveillance for infection, testing everyone at a regular interval for the presence of the SARS-CoV-2 virus. People were tested due to symptoms or close contact with someone who had COVID-19. However, there is a subset of the Johnson and Johnson trial data where they *did* look at asymptomatic infections (very exciting!). More on that in a bit.
Another way to represent the information in the graphs shown above is in the table below (for Johnson and Johnson). In this table, they look at vaccine efficacy for how well the vaccine does against moderate to severe COVID-19. They specifically looked at those who developed infection at least 14 days and 28 days after vaccine/placebo injection. That’s important because we can see infections in the first 14 days that are coincidental - people were exposed at or around the time of the injection before the immune system had a chance to see and respond to the vaccine. Let’s focus on the right side of the table (onset at least 28 days) in the row for “all participants.” The Ad26.COV2.S is the vaccine group. The trial groups are nearly equal, but not perfect. In the placebo group 193 people were diagnosed with moderate to severe/critical COVID-19 out of 19,179 participants (or 1.0% of the placebo population). For those who were vaccinated, there were 66 cases out of 19,306 (or 0.34% of the vaccine population). Efficacy can be calculated as risk for the unvaccinated group (1%) minus risk for the vaccinated group (0.34%) divided by risk for the unvaccinated group (1%). That gets us to the 66% vaccine efficacy number that’s highlighted below. In other words, the vaccine group saw 66% fewer infections than the placebo group. You can read more about calculating vaccine efficacy here.
Another cool thing in this table is that the efficacy is consistent for older adults compared to younger adults. That wasn’t the case for Moderna where efficacy was 86.4% in adults 65+ compared to 95.6% in younger adults.
Let’s talk about the elephant in the room. Yes, the Johnson and Johnson vaccine does not prevent infection as well as the Pfizer and Moderna vaccines. However the infections that do happen appear to be more mild than what we see for the unvaccinated population. And that’s a good goal for vaccination too - to bring this pandemic’s severity down to something similar to the common cold. We don’t close schools or businesses for outbreaks of the common cold. This vaccine may not be as great as the other two (a good reminder of just how lucky we are to have them) but it is “good enough” to accomplish the goal of ending the pandemic. Look at what I mean in the table below. I discussed a similar table in Wednesday’s newsletter. If we look at the efficacy of preventing hospitalization, then the Johnson and Johnson vaccine does quite well. At 14 days the efficacy is 93.1% and at 28 days the efficacy is 100%.
A lingering question that is unanswered by this clinical trial is how well the vaccine does at preventing long-haul COVID-19, the long term complications that some are experiencing for months after infection. It should be noted that many of the long-haul COVID-19 patients had mild infections. So of the mild infections that happened in the Johnson and Johnson trial, how many of those patients developed long term symptoms? A reminder, an EUA is not the same thing as full FDA approval. These manufacturers will all continue to collect and submit efficacy and safety data to the FDA for about 2 years until full approval is granted. So we may learn more about long-haul COVID-19 from Johnson and Johnson at a later date.
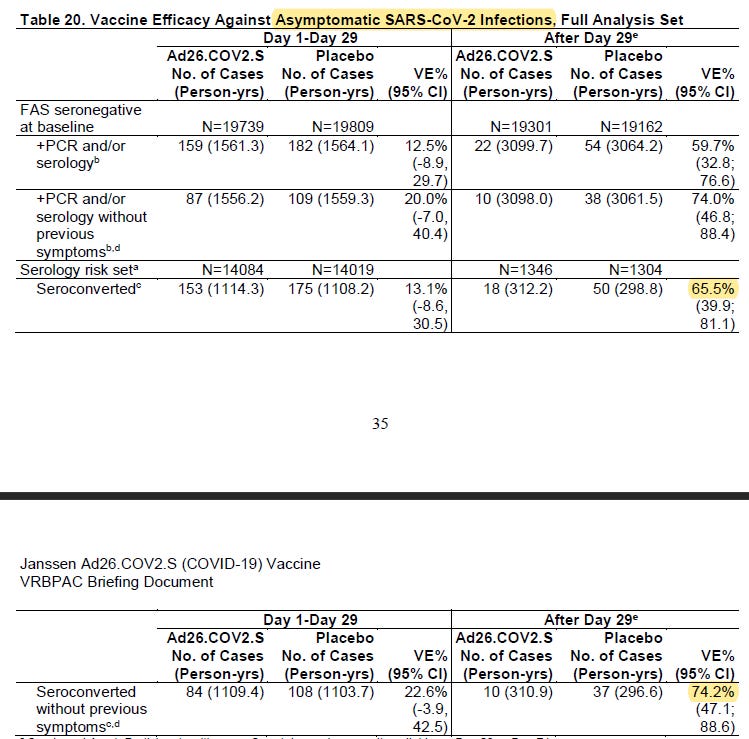
Something REALLY cool about this trial by Johnson and Johnson is that they looked at how well the vaccine did at preventing asymptomatic infection. This is a really big deal because an estimated 40% of cases during the pandemic are asymptomatic and these people unknowingly contribute to ongoing disease transmission in their communities. So this table is complicated. Let me explain what they did. The vaccine only includes the gene for the SARS-CoV-2 spike protein (S protein). Antibodies are proof of immune system response, but they needed to distinguish from the antibodies they expected the vaccine to produce (against the spike protein) and those due to natural infection. They could test for antibodies due to natural infection by looking for antibodies to the nucleocapsid (the protein that surrounds the SARS-CoV-2 genome). This is called the N protein for the purposes of this discussion. To recap, they were looking for antibodies to a part of the virus that they didn’t provide in the vaccine. The only way the immune system could make those antibodies was if the person had been exposed to and responded to the SARS-CoV-2 virus in its original form. At the start of the study (day zero) they confirmed that no one had antibodies to the virus. I want us to focus on the bottom half of the table that unfortunately didn’t fit onto one page. Start where it says “serology risk set.” This was the group for whom they had a serology test result on day 29 or 71. All the people who had a positive antibody test for the N protein (in response to natural infection) are in the row where it says “seroconverted.” So this is the population who tested positive for N protein antibodies whether they had symptoms or not. For up to day 29, the vaccine showed 13.1% in this group, but 65.5% after day 29.
Next, let’s look at those who produced N protein antibodies but didn’t display any symptoms (“seroconverted without previous symptoms). These are asymptomatic infections. After 29 days, the vaccine group had 74.2% fewer asymptomatic infections than the placebo group. This is information that we don’t have for the other vaccines. We didn’t initially have data to exclude the possibility that people vaccinated with the Pfizer or Moderna vaccines could potentially transmit the virus to others, even if they were asymptomatic. Johnson and Johnson didn’t look at transmission after vaccination per se, but it’s a lot harder to transmit disease if you don’t have it and asymptomatic carriers were the possibility we were most worried about. So an added benefit of the Johnson and Johnson vaccine is this reduction of asymptomatic infection.
Vaccine Q&A
Why did we have to wait so long for the EUA for the Johnson and Johnson vaccine?
Johnson & Johnson submitted their application for Emergency Use Authorization (EUA) on 04Feb2021. The committee that makes the recommendation for EUA is meeting today (26Feb2021) and some are upset at what they perceive as a lack of urgency. Shouldn’t these folks have met the next day?
Here’s the deal. A similar vaccine has been approved for use by the European Union (for Ebola) but this was the first adenovirus vector DNA vaccine to make it to this point in the US. Trust me, this is something where we should want the advisory panel to carefully sift through all of the clinical trial data with a very skeptical lens as this is a brand new vaccine technology for the US. In addition, Johnson and Johnson didn’t take the Operation Warp Speed incentives that paid for vaccine manufacturing during development. Part of that program’s requirements was that manufacturers submit their clinical trial data early and often to FDA, ahead of their EUA application. Since J&J didn’t take that money, they didn’t submit data until 04Feb2021. So the advisory panel had to go through ALL the data at that point. It might seem like unnecessary delay, but it is important for ensuring that they recommend a beneficial and safe product for use in humans. In addition, it is a critical step in maintaining public trust that this vaccine was well-vetted.
Vaccines, pregnancy and fertility
There is a big disinformation effort going around on the internet right now that aims to convince women of childbearing age that they should not get the COVID-19 vaccine due to unspecified impacts on fertility. However, there are no data to support this argument. We do have some data in laboratory animals for both the Johnson and Johnson and Moderna vaccines. Below is the section where FDA summarizes the findings for the Johnson and Johnson vaccine. It can be found on page 52 of the briefing document.
Rabbits are a good animal for this kind of study because their gestation period is much shorter than humans (about 4 weeks compared to 40 weeks in humans). They can also test lots of rabbits (high sample size is a good thing) whereas testing in pregnant women or those who want to become pregnant is ethically challenging. As a result, a lot of the data we have on pregnant women is anecdotal - information gathered after the fact such as women who became pregnant during the study or chose to be vaccinated regardless. Anyway…they tested rabbits before they were mated and during gestation (while the rabbit babies were developing in utero) using a dose of the vaccine that was 2 times higher than the dose used in humans. And they found no impacts on the rabbits’ ability to reproduce, no birth defects and the rabbit babies developed normally after birth. This would predict that using half that dose in women of childbearing age would be similarly safe. In the Moderna study (page 45 of its briefing document) they used the same dosage as recommended for humans but used rats for reproductive analyses (gestation period ~3 weeks).
Disinformation efforts sometimes take advantage of situations where it’s hard to prove an unknown in an emotionally charged context (i.e. pregnancy). They don’t have to present any evidence that an adverse reaction actually occurs, they’re arguing that you don’t know that it doesn’t. And miscarriage and birth defects are understandably scary. The situation in pregnant women is complicated by the fact that we are generally averse to experimenting on pregnant women. But we do have laboratory animal studies (discussed above) and data is being continually gathered on pregnant women who have received the Pfizer and Moderna vaccines. So far, the animal studies suggest an excellent safety profile with respect to reproduction. Study of pregnant women who choose to be vaccinated is planned for the continuing Johnson and Johnson trials. Because of the longer gestation period for humans (40 weeks), it takes longer to monitor and gather data.
The American College of Gynecologists and Society of Maternal-Fetal Medicine have issued a joint statement supporting the use of the COVID-19 vaccines in pregnant women if they choose to be vaccinated. They cite the excellent safety profile in laboratory animals and the considerable risk that full COVID-19 infection poses to pregnant women. They offered a more inclusive summary and recommendation for women planning to become pregnant and those who are lactating in December. A compelling argument for vaccinating lactating mothers is that they pass antibodies through breastmilk, providing passive immunity against COVID-19 to their newborn/infant.
There is some evidence that infection with the full blown SARS-CoV-2 virus (not the vaccine) can impact fertility but it’s in men, not women, since the virus receptor is present in some of the tissues of male reproductive anatomy. So if impacts on fertility are a concern, the goal should be to prevent COVID-19 infection in the first place. We can do that safely by using the vaccine.
I got vaccinated. Now what can I do?
One of the biggest incentives for getting vaccinated is a speedier return to pre-pandemic life. Many people haven’t seen certain family and friends in nearly a year. To say that the pandemic has been disruptive is a huge understatement.
During development and clinical trials for the Pfizer and Moderna vaccines they didn’t study whether these vaccines interrupted transmission - something that is most likely to occur due to asymptomatic carriage and transmission of the virus. With COVID-19 disease burden being as high as it was in December when these were approved, I think we can appreciate why answering that question matters. We are starting to see some studies that suggest the vaccines reduce viral load in those who are infected after vaccination. In addition, Moderna provided supplemental preliminary data that demonstrated that vaccine recipients who had received 1 dose had 66% fewer asymptomatic infections than those in the placebo group when they came in at the scheduled time for their 2nd dose (diagnosed by PCR). We don’t know yet whether the second dose takes that number down even further. And I talked about the J&J impact on asymptomatic infection in the top section of this newsletter. So the data are promising that the vaccines do limit transmission, but we need more data to confirm.
Between the vaccine and adherence to public health recommendations for masking, social distancing, etc, cases should go down in a big way. But until we get a lot of people vaccinated, our primary tools against this pandemic remain the less glamorous ones that we are all tired of doing. I think the recommendation that vaccinated persons continue to mask and social distance has two motivations: (1) until we know for sure that the vaccine prevents asymptomatic transmission, this is the safest course of action and (2) we need to continue to normalize adherence to public health guidance in our communities until more are vaccinated. If you suddenly see that fewer people around you are wearing masks, you might be more likely to take yours off too. We don’t have a way to know if the maskless strangers around us are vaccinated or just careless. So for that reason, we need to continue to be good examples until a greater portion of the population is vaccinated.
However, I think if a small group of adults who have all been fully vaccinated want to gather for dinner in one of their homes, then it is likely safe for them to do so. But keep it small. I want to caution you against fully reverting back to pre-pandemic social interactions. Start small and ease into it, prioritizing the social contacts who matter the most (ideally just a few new vaccinated people until we know more).
As more people are vaccinated we can start to forecast some ways that different situations will present. To be clear, we aren’t there yet. But this is what might be possible soon if we can confirm that the vaccines interrupt transmission. If you’re planning a mixed gathering where some have been vaccinated and others haven’t, then those who haven’t been vaccinated should still adhere to the existing public health recommendations including masking and social distancing, ideally outdoors. If you’re at a gathering where no one has been vaccinated, then everyone should adhere to the public health recommendations we have now. And I might argue that an indoor gathering is off limits entirely.
Things get more complicated when children are factored in, since none of them are currently eligible for vaccination. As I’ve stated before, things will not be “normal,” until they are also vaccinated. But we can probably expand our “bubble” of trusted contacts after vaccination. I’ve had a number of healthcare workers write to me who still worry about bringing COVID-19 home to their kids even though they (the parents) have been vaccinated. We don’t have enough evidence to know for sure, but the evidence that suggests transmission is less likely after vaccination is accumulating. I don’t know if that provides reassurance, but perhaps it provides hope.
Can we see the grandparents now?
Nothing we do in life is risk free, and especially during a pandemic. But if the grandparents have been fully vaccinated (2 weeks since 2nd dose), then things are safer than they were a few months ago. If you have been fully vaccinated also, even better. In fact, that’s my preference, that you wait until every adult involved is vaccinated. The kids are still ineligible for vaccines, but I suppose that if all the adults are vaccinated then the kids are the ones left with the highest risk. Their risk isn’t super high to begin with, but it isn’t zero either. That’s a decision you’ll need to make in the context of your own family’s unique situation.
In my family’s situation, my father-in-law’s birthday is coming up soon. By that time the in-laws will be >2 weeks past their 2nd dose as will my husband and I. We are planning to go see them at their home to celebrate (with our two children) and will probably hug the grandparents for the first time in over a year. But we may prioritize that close encounter outside and we’ll have the kids wear their masks when indoors.
More vaccine Q&A next Friday! Stay tuned!
Georgia updates
Vaccines
In case you haven’t heard, the Governor has announced they are doing away with phases and are just going to keep a running list of who is eligible. Starting March 8, adults with developmental disabilities and the parents/caregivers of children with developmental disabilities, pre-K-12 teachers and staff will be eligible for vaccines. This population is estimated to be about 1 million people in Georgia. The expanded eligibility does not yet include faculty and staff in higher education (i.e. college and universities). Later in March, the Governor expects to expand eligibility to additional populations with severe underlying medical conditions.
I’m REALLY happy for the people who will be eligible shortly. There is no word on expanding eligibility to other essential workers (i.e. grocery, food service, transportation, utilities, etc). The recommendations laid out by the Advisory Committee on Immunization Practices that sought to balance saving lives and protecting essential workers with the greatest risk of exposure don’t appear to be part of vaccine prioritization in Georgia. We are asking essential workers who can’t work from home to take all of the risk with little hope of reward for their sacrifice and effort over the past year. For these groups impacted by the change, the best hope is that vaccine supply increases dramatically and soon.
Here are some ways to register for a vaccine appointment:
Central registration through DPH for an appointment at a Georgia health department (this is new, yay!)
Pre-register for an appointment through a GEMA mass vaccine event. If you have already registered here, you don’t need to do anything else. If you qualify under the expanded criteria, your eligibility will be updated automatically.
Vaccine appointments through retail pharmacy chains
Testing
Today there were 27,773 newly reported PCR test results and 14,652 newly reported antigen tests through Electronic Laboratory Reporting (ELR). We’ve seen a lot of testing reported outside of ELR that can make calculation of percent positive rate less reliable. But that problem has been resolved today, with 88% of PCR tests and 91% of antigen tests reported through ELR. Of the PCR tests, 7% were positive and for antigen tests the positivity rate was 7.7%. It was a mid-range day for PCR test output. We don’t have enough history yet on antigen cases to know what is “typical” for output.
Cases
The school aged surveillance data report is scheduled to be released today but hasn’t been posted yet by DPH.
Today was notable in that Georgia crossed 1 million COVID-19 infections since the start of the pandemic. Today there was a net increase of 2208 newly reported PCR cases and 1226 newly reported antigen cases for a combined total of 3434. The updated statewide total is 1,000,822.
One thing that’s giving me a bit of anxiety is that we’re seeing the 7-day case rate start to level and increase again for the Atlanta metro and for nonrural counties outside of the Atlanta metro. For Atlanta counties (Fulton and DeKalb) the 7-day case rate per 100,000 has risen 22% in the past week. The suburbs have had more of a plateau, with only a 4% increase in the past week. Case rate is flat for nonrural counties over the past week.
We know that the more transmissible virus variant that rose to dominance in the United Kingdom is in Atlanta and other areas in the state. Atlanta hasn’t seen an increase that was sustained like this since the descent from the winter surge peak began. It’s still too soon to know if this increase is due to the variant or a problem at all yet. But this is the part of my daily data analysis right now where I hold my breath before pushing “enter,” hoping that the trend has reversed. It hasn’t yet. The state case rate has leveled off at a rate that is 152% higher than the rate on 02Oct2020, the pre-surge baseline. I’m hopeful we’ll see the decline continue since we saw similar plateaus in the descent that followed the summer surge. We’ll revisit this on Monday.
Hospitalizations
There was a net increase of 174 new hospital admissions for COVID-19 and 51 admissions to the ICU. The admissions to the ICU are high compared to where we’ve been over the past month. The last time we saw a number this high was 09Feb and prior to that it was 21Jan. It is uncommon for Georgia to have this many ICU admissions in a day.
According to the Department of Health and Human Services, there are 2634 patients in the hospital for COVID-19 in Georgia. The HHS data set includes pediatric patients and suspected COVID-19 infections for those who don’t have laboratory confirmation at the time of admission. It is therefore a more inclusive data set than the one provided by the state of Georgia. At 2634, Georgia hasn’t seen a number this low since 08Dec2020. It’s definitely good news. Twelve of the 14 hospital regions are in the green or yellow zone for ICU bed use and COVID-19 patient census is down, so I hope that hospital staff are able to get some much deserved rest. Let’s make the winter surge the last one for Georgia. Keep up the mask wear and social distancing until more of the population can be vaccinated.
Deaths
There was a net increase of 18 confirmed COVID-19 deaths and 2 probable deaths (combined total of 20). This is a LOW count for a weekday, especially when we’ve seen many 100+ daily counts on weekdays for the past month. We haven’t seen a low count like this on a weekday since the winter holidays when reporting was really delayed. So I want to celebrate a low count for today, but I want to also temper that enthusiasm with the possibility that this could just be a weird day for reporting. Let’s see what happens through Tuesday of next week before we launch the confetti.
That’s it for today. Have a great (socially distanced) weekend!
References
Johnson and Johnson: https://www.fda.gov/media/146217/download
Moderna: https://www.fda.gov/media/144434/download
Pfizer: https://www.fda.gov/media/144245/download
https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html
https://www.acog.org/news/news-releases/2021/01/acog-and-smfm-joint-statement-on-who-recommendations-regarding-covid-19-vaccines-and-pregnant-individuals
https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
https://rep.bioscientifica.com/view/journals/rep/161/2/REP-20-0523.xml
https://www.medrxiv.org/content/10.1101/2021.02.06.21251283v1
https://www.fda.gov/media/144453/download
https://healthdata.gov/dataset/covid-19-reported-patient-impact-and-hospital-capacity-state
https://dph.georgia.gov/covid-19-daily-status-report
https://covid-gagio.hub.arcgis.com/
Georgia COVID-19 Updates is a free newsletter that depends on reader support. If you wish to subscribe please click the link below. There are free and paid options available.
My Ph.D. is in Medical Microbiology and Immunology. I've worked at places like Creighton University, the Centers for Disease Control & Prevention and Mercer University School of Medicine. All thoughts are my professional opinion and should not be considered medical advice.